Ogni Stazione Radio di Bordo è dotata di un sistema di alimentazione autonomo in grado di garantire il funzionamento degli apparati in caso di guasto e /o mancanza di tensione elettrica nella Nave.
Questo sitema supplementare è costituito da batterie tipo piombo-acido che a seconda della combinazione e degli apparati di Bordo possono essere configuarate in serie o parallelo tanto da fornire una tensione di 12/24Volt con capacità adeguata al fine di garantire il funzionamento per diverse ore (almeno 6).
E’ compito dell’RT mantenerle in piena efficienza verificando giornalmente la tensione di mantenimento e se necessario ricaricarle con il sistema automatico di Bordo. Uno degli strumenti conosciuto da tutti gli Operatori è il densitometro/densimetro che consente una verifica della carica in modo chimico e non elettrico, cioè andando a misurare la densità dell’elettrolita.
Qui sotto riporto le consegne da tenere secondo un manuale di recente pubblicazione,
MARINE RADIO OPERATORS HANDBOOK – AUSTRALIAN MARITME COLLEGE
SECTION 10 CARE AND MAINTENANCE OF BATTERIES
53. LOCATION OF BATTERIES
53.1 The location of a battery supplying marine radio
equipment should be chosen to ensure that, as
far as practicable, the battery is:
>> protected from the elements;
>> readily accessible for routine maintenance;
>> located reasonably close to the transceiver;
>> located as high in the vessel as practicable;
>> well ventilated to dissipate the hydrogen gas
produced (if located within a wheelhouse or
other compartment, venting to the outside
may be necessary);
>> not located with other items of equipment
that could, in heavy weather, fall across the
battery and cause short-circuiting; and
>> not located in the same compartment as
a different type of battery, for example,
alkaline cells. ◆•
54. CONSTRUCTION OF LEAD ACID CELLS
54.1 Lead acid cells have a voltage of 2 volts per
cell, regardless of size. Larger size cells will
supply higher current than smaller cells, or the
same current for longer periods. The ability of
a cell to produce current for a period of time
is known as the cell’s capacity and is usually
measured in ampere-hours (Ah), or with batteries
designed for motor vehicle use, as ‘cold cranking
amps’ (CCA). ◆•
54.2 A chemical combination of lead and lead peroxide
plates and the sulphuric acid in the electrolyte
(the liquid solution within the cell), produces
a voltage difference between the plates. This
voltage difference allows a current to flow through
any load, such as a radio, connected across the
battery terminals and is called direct-current or
‘dc’. ◆•
54.3 When the acid in the electrolyte or the material in
the plates is used up, the voltage no longer exists
and current cannot flow. At this point, the cell is
said to be discharged or “flat”. ◆•
54.5 This situation is reversible by passing a current
in the opposite direction. This process reverses
the chemical reactions in the cell and is known as
charging.◆•
55. CONNECTION OF LEAD ACID CELLS
55.1 Cells may be connected in series, that is, the
positive terminal of one cell to the negative
terminal of another, to produce higher voltages.
Three cells connected in series will give a “battery”
of 3 x 2 volts = 6 volts; six cells connected in series
will give a “battery” of 6 x 2 volts = 12 volts. ◆•
55.2 Most modern lead-acid batteries are supplied in
6 or 12 volt combinations and may themselves
be connected in series to provide the required
output voltage, for example, two 12 volt batteries
connected in series will produce a voltage of 2 x
12 volts = 24 volts. ◆•
29
55.3 Connection of lead-acid batteries in parallel, that
is positive terminal to positive terminal, negative
terminal to negative terminal, will produce the
same output voltage as a single battery, but
the ability to supply current (capacity) will have
been lengthened. For example, two batteries
each supplying 12 volts with a capacity of 60
ampere-hours, when connected in parallel
will provide a voltage output of 12 volts with a
capacity of 120 ampere-hours. ◆•
56. ESSENTIAL BATTERY MAINTENANCE
56.1 The functioning of radio equipment is dependent
on power supplied by the battery. If it is to
provide adequate performance in the event of
an emergency, regular and careful maintenance
is required.
56.2 A battery’s service life also depends on the
manner in which it is treated.
56.3 To ensure the best performance from a battery
it is important that a battery:
>> is kept clean, dry and free from terminal
corrosion;
>> has the electrolyte kept at the correct level;
and
>> is kept correctly charged. ◆•
Positive
Negative
Positive
Negative
CHAPTER 4 – Power Supplies
3 0 MARINE RADIO OPERATORS HANDBOOK – AUSTRALIAN MARITME COLLEGE
57. BATTERY CLEANLINESS
57.1 A battery top should be kept clean. A dirty battery
top may hold spilt electrolyte on its surface thereby
providing a path for the electrical current to leak
away. It is important to keep the outside surfaces
of a battery dry and free of contamination. ◆•
57.2 Corrosion forming on terminal clamps may
seriously affect, or even prevent, the ability of
the battery to supply current. Corrosion will
be evident by the formation of a white-green
powder between the battery terminals and the
terminal clamps. In this situation, the terminal
clamp should be removed and both it and the
terminal post cleaned. ◆•
57.3 To minimise the likelihood of corrosion, terminal
posts and clamps should be lightly smeared with
Vaseline™ or petroleum jelly. ◆•
58. ELECTROLYTE LEVEL
58.1 The level of electrolyte inside a battery is
important. As a result of the chemical action
inside a battery, water is lost. This should be
replaced with distilled or demineralised water.
◆•
58.2 Seawater must not be used under any
circumstances.
58.3 The level of the electrolyte should be maintained
at approximately 10 mm above the plates unless
otherwise specified by the manufacturer. ◆•
58.4 If the electrolyte level is too high, it may overflow
during charging providing an unwanted discharge
path. If the electrolyte is too low, the plates are
exposed to the air and permanent damage and
loss of capacity may result.
58.5 It may be noticed that a battery that is nearing
the end of its useful life will require more frequent
topping-up than has been previously necessary.
58.6 Low-maintenance batteries will require infrequent
topping-up. Maintenance-free batteries may
require none at all.
59. CORRECT CHARGING
59.1 To provide the best service, a battery must
be correctly charged. Both overcharging
and undercharging can seriously affect its
performance. ◆•
59.2 On small vessels the usual means of charging the
radio battery will be an alternator or generator
attached to the vessel’s engine. An associated
regulator, which reduces the charging current as
necessary, should prevent overcharging.
59.3 Vessels that are used frequently (say, several times
each week) should have no problem maintaining
a fully charged radio battery. However, on vessels
that are used relatively infrequently (once every
few weeks), it is likely that during storage even
a battery that starts as fully charged, will selfdischarge
and go flat.
59.4 For safety reasons, it is important that the vessel
owner is able to determine the general condition
of a battery and its ability to supply current over
a period of time (its capacity). An indication of the
level of charge in a battery may be obtained by
either:
>> measuring the specific gravity of the
electrolyte; or
>> measuring the on-load terminal voltage. ◆•
60. MEASURING THE SPECIFIC GRAVITY
60.1 The specific gravity, also called the relative density,
of the electrolyte (the liquid inside the battery)
varies proportionally with the amount of charge
in the battery. It is highest when the battery is
fully charged and lowest when the battery is fully
discharged or flat. It follows that the amount
of charge in a battery can be determined by
measuring the specific gravity of the electrolyte.
◆•
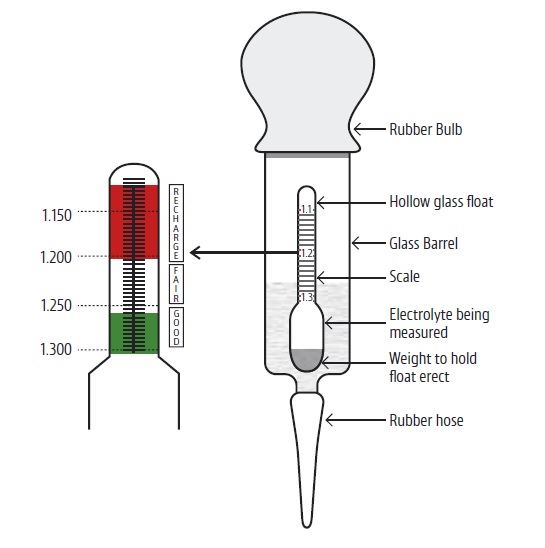
60.2 A simple, inexpensive device called a hydrometer
is used to measure specific gravity. ◆•
(See diagram on Page 31).
60.3 In general, for a fully charged battery, the specific
gravity should measure about 1.250. Half charge
will be indicated by a reading of 1.200 and fully
discharged by 1.150. All cells in a battery should
indicate a similar specific gravity. A variation of
more than about 0.025 will indicate a faulty cell
and the battery should be replaced. ◆•
60.4 Specific gravity readings should not be taken
immediately after topping-up a cell as the added
water will float towards the top of the cell and
give a false reading. Charging for thirty minutes
or more after topping-up will mix the electrolyte
and allow accurate readings.
31
60.5 Batteries which have cells where specific gravity
readings fail to rise, or respond poorly to adequate
charging, should be replaced.
61. MEASURING THE ON-LOAD TERMINAL VOLTAGE
61.1 Measurement of the terminal voltage when a
battery is supplying current to a load, such as
a radio, will also provide an indication of the
amount of charge in a battery. This measurement
is known as the on-load terminal voltage. ◆•
61.2 For a 12-volt battery, the on-load terminal
voltage should not fall below approximately
11.4 volts while transmitting. If the voltage does
fall significantly below this figure, the battery
requires charging. If after charging, the on-load
terminal voltage still falls significantly below 11.4
volts, it is an indication of a faulty cell and the
battery should be replaced.
61.3 Measuring of the off-load (that is, when the
battery is idle) terminal voltage of a battery is a
poor indication of its condition. ◆•
62. LOSS OF CAPACITY
62.1 A battery will suffer a gradual loss of capacity
during its life. This is inevitable and the battery
should be replaced when the capacity loss
becomes significant.
62.2 Many lead-acid batteries have a commercial life
of only two to three years.
62.3 However, the useful life of a battery can be
considerably shortened by:
>> operating a battery in a low state of charge for
long periods;
>> allowing a battery to stand in a discharged
state for long periods;
>> leaving a charged battery for long periods
without periodic charging; and
>> overcharging. ◆•
63. BATTERY HAZARDS
63.1 There are two hazards associated with lead-acid
batteries that ship station operators should be
aware of:
>> the risk of explosion; and
>> the risk of chemical burns. ◆•
63.2 As a result of the chemical process occurring
within the cells of a battery during charging,
Hydrogen Gas is produced. When mixed with air,
this can form a highly explosive mixture which can
be ignited by a naked flame, a lighted cigarette, or
a spark. The spark caused by breaking or making
an electrical connection in the vicinity of the
charging battery may be sufficient to ignite the
hydrogen-air mixture. Batteries should be located
close to the radio equipment and placed in a well
ventilated container or locker ◆•
1.3
1.2
1.1
RECHARGE
FAIR
GOOD
Rubber Bulb
Hollow glass float
Glass Barrel
Scale
Electrolyte being
measured
Weight to hold
float erect
Rubber hose
1.150
1.200
1.250
1.300
CHAPTER 4 – Power Supplies
3 2 MARINE RADIO OPERATORS HANDBOOK – AUSTRALIAN MARITME COLLEGE
63.3 If using metal tools to work on battery connections,
extreme care must be taken to ensure that
terminals are not short-circuited. ◆•
63.4 The electrolyte in battery cells contains Sulphuric
Acid. It is sufficiently concentrated, particularly
just after charging, to damage eyes, skin or clothes
if spilt or splashed. Immediate and prolonged
application of running water is recommended to
minimise its effect. ◆•
63.5 It is recommended that eye protection, gloves,
etc. be worn when a person is carrying out
maintenance on batteries. Batteries should not
be topped-up whilst on charge. ◆•
64. MAINTENANCE FREE BATTERIES
64.1 Maintenance Free: Maintenance free Lead – Acid
or Gel type batteries are becoming increasingly
available to mariners. Users of these types
of batteries are recommended to follow the
manufacturer’s guidelines in ascertaining the
condition of the battery before replacement.
On vessels where it is mandatory to carry an
independent emergency means of electrical
supply, for communications equipment, it may
also be a requirement to replace ‘maintenance
free’ batteries after a short operational
period of 1 year.